Abstract
| |
Males
(n = 10) |
|
Females
(n = 10) |
||||
| Age (y) | 18.3 | ± | 2.2 | |
18.6 | ± | 2.6 |
| Height (cm) | 178.2 | ± | 7.3 | |
167.1 | ± | 5.5# |
| Body weight (kg) | 65.7 | ± | 9.8 | |
57.8 | ± | 4.8* |
| Body Mass Index (kg/m | 20.6 | ± | 1.8 | |
20.7 | ± | 1.1 |
| Training experience (y) | 10.0 | ± | 4.2 | |
12.7 | ± | 3.5 |
| |
Males
(n = 10) |
|
Females
(n = 10) |
||||
| Heart rate (beats∙min–1) 50 m front-crawl | 174.8 | ± | 11.4 | |
176.3 | ± | 10.9 |
| 100 m front-crawl | 175.2 | ± | 11.1 | |
176.7 | ± | 10.8 |
| 200 m front-crawl | 177.5 | ± | 3.4 | |
176.9 | ± | 4.4 |
| Mean time (s) 50 m front-crawl | 30.1 | ± | 1.3 | |
32.2 | ± | 1.1* |
| 100 m front-crawl | 63.9 | ± | 3.2 | |
69.2 | ± | 1.8* |
| 200 m front-crawl | 140.5 | ± | 7.4 | |
151.1 | ± | 4.6* |
| Fatigue (Borg scale) 50 m front-crawl | 16.2 | ± | 1.1 | |
16.0 | ± | 1.2 |
| 100 m front-crawl | 16.4 | ± | 1.5 | |
16.3 | ± | 1.3 |
| 200 m front-crawl | 16.1 | ± | 1.6 | |
16.3 | ± | 1.4 |
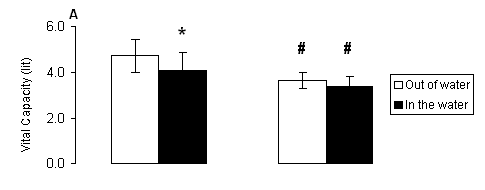
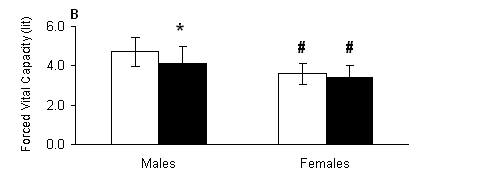
Fig. 1. A) Vital capacity (VC) and B) forced vital capacity (FVC) measured out of water (open bars) and in the water (solid bars) in male and female swimmers (mean ± SD). *, Significantly different from the respective value measured out of water within the same group (P < 0.05). #, Significantly different from the respective value measured in males at the same condition (P < 0.05).
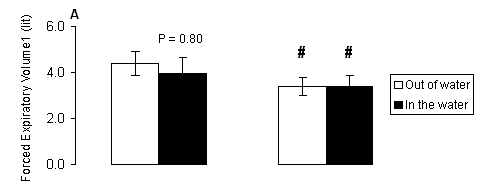
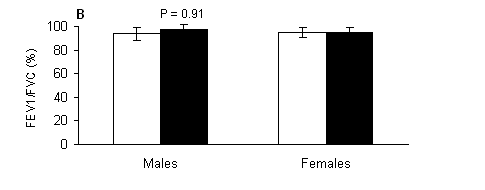
Regarding forced expiratory volume at one second, there were significant main effects of gender (P < 0.001), marginally no significant main effects of treatment (P = 0.069) and no significant treatment-by-gender interaction (P > 0.05). In males, FEV1 (Fig. 2A) had trend (P = 0.080) to be lower in the water compared to the respective value measured out of the water. Females (Fig. 2A) had lower FEV1 compared to respective values of males both out of (P < 0.001) and in the water (P < 0.01). No significant (P > 0.05) main effects of treatment, gender or treatment-by-gender interaction were observed for FEV1/FVC ratio (Fig. 2B).
Fig. 2. A) Forced expiratory volume at one second (FEV1), and B) indicator Tiffeneau (FEV1/FVC) measured out of water (open bars) and in the water (solid bars) in male and female swimmers (mean ± SD). #, Significantly different from the respective value measured in males at the same condition (P < 0.05).
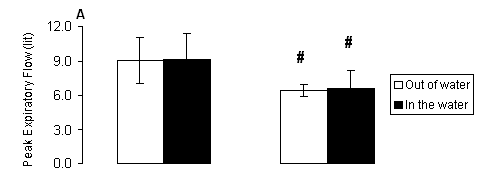
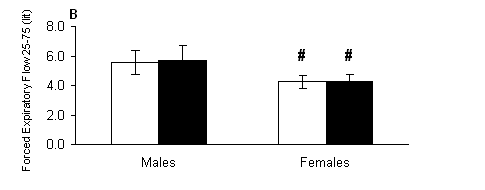
With regard to peak expiratory flow, there were only significant main effects of gender (P < 0.001) but no significant main effects of treatment or treatment-by-gender interaction (P > 0.05). Females (Fig. 3A) had lower PEF compared to respective values of males both out of (P < 0.01) and in the water (P < 0.01). Similarly for forced expiratory flow rate at 0.25-0.75 sec, there were only significant main effects of gender (P < 0.001) but no significant main effects of treatment or treatment-by-gender interaction (P > 0.05). Females (Fig. 3B) had lower FEF 25-75 compared to respective values of males both out of (P < 0.001) and in the water (P < 0.001).
Fig. 3. A) Peak expiratory flow (PEF) and B) forced expiratory flow rate at 0.25-0.75 sec (FEF 25-75) measured out of water (open bars) and in the water (solid bars) in male and female swimmers (mean ± SD). #, Significantly different from the respective value measured in males at the same condition (P < 0.05).
DISCUSSION
Our data indicate that during the testing in the water at rest, the functional capacity of the respiratory system is limited in males only, as shown by the decreased VC, FVC and FEV1. As expected, females had lower values in all parameters compared to respective values of the males, but the pulmonary function in female swimmers was not affected in the water at rest. More specifically, as reflected by the changes in the rates of respiratory parameters in the water compared to values measured out of the water, in males there was a decrease in VC by 13.32%, FVC by 13.14%, FEV1 by 10%. In females, there was a decrease in VC by 7.68%, FVC by 5.30% and FEV1 by 5.90%. The changes in all other respiratory parameters were small and insignificant.
For same volumes of water and air, the water has bigger mass. The pressure of water is felt by the swimmer and influences mainly the points that contain air, such as are the thorax, the lungs, the ears and elsewhere. According to the literature, the thorax found hardly under the surface of water is little pressed in such degree, that the VC is decreased at 9% roughly. Breathing, mainly the inhalation phase, may be affected as the thorax presses on the opposite direction to the pressure exerted by water. Still the hydrostatic pressure presses the surface veins, thus resulting in distribution of blood which is increased in the centre and decreased in the region.
Furthermore, the hydrostatic pressure results in a restricted inspiratory force, thus reducing lung capacity and VC. A reduction in VC by 3-9 % has been reported when individuals are immersed to the level of the xiphoid process (Agostoni, Gurtner, Torri, & Rahn, 1966). Decreases in VC, functional residual capacity and expiratory reserve volume have been attributed to the combined effects of hydrostatic chest compression and increased intrathoracic blood volume. Similar changes occur in VC and expiratory reverse volume, the progressive increase in chest compression being represented by a rightward shift of the volume-pressure curve (Chu & Rhodes, 2001). The level of immersion, therefore, is a key influence in determining alterations in pulmonary parameters.
The swimmers’ performance was assessed in the 50, 100 and 200 m front crawl tests. Furthermore the swimmers’ perceived exertion rate was classified on the Borg scale at the end of 50, 100 and 200 m swimming performance tests. In all three swimming performance tests and for both male and female swimmers, the subjective perception of the effort according to Borg scale was about 16, which is considered high. Specifically, the swimmers of male rated their level of effort at 16 and of female at 15. The level of these rates corresponds to about 80-95% of the HR max of the particular age, which is considered a level of sub-maximal intensity (McArdle, Katch, & Katch, 2000). The HR max recorded immediately after the swimming performance tests corresponds to 80-95% of HR max according to the age of the swimmers of the sample in question, thus confirming the values of Borg’s perceived exertion scale. This particular level of intensity is deemed capable of bringing about an improvement in the anaerobic capacity. Even though exercise taken for 10 to 20 minutes at the 80-95% level of intensity leads to an improvement of the anaerobic capacity (McArdle et al., 2000).
According to Bye, Farkas, and Roussos (1983) the respiratory muscles are subject to fatigue during high intensity exercise (>85% VO2 max). Lomax and McConnell (2003) demonstrated that a single 200 metre front-crawl swim corresponding to 90-95% of race pace was sufficient to induce inspiratory muscle fatigue. It is possible that exercise training results in several positive adaptations that could affect respiratory muscle fatigue (Volianitis et al., 2001). Appropriate exercise intensity is an important consideration for the development and maintenance of performance during land-based training (Wenger & Bell, 1986). Proper training intensity seems to be even more significant because the circulatory responses of running in deep water differ from those of on-land running (DiPrampero, 1986; Wenger & Bell, 1986; Brown, Chitwood, Beason, & McLemore, 1996). In water that is chest deep or higher, hydrostatic pressure exerted by the water causes a redistribution of blood volume away from the extremities, resulting in greater amounts of blood in the thorax region (Christie et al., 1990). The central shift in blood volume increases the amount of blood ejected from the heart per beat (stroke volume), thus producing a compensatory reduction in heart rate (HR) during deep water exercise. Studies report HR responses to be approximately 10-12 beats lower than those achieved during land running at matched sub-maximal intensities (Ritchie & Hopkins, 1991; Frangolias & Rhodes, 1995).
The effect of water in the human organism is also the alleviation of cardiac frequency from the moment that the body of the swimmer remains for more from 10 seconds under the water surface. The alleviation becomes bigger at the moment that the body is sunk entirely and the swimmer keeps air in the lungs, while he remains underwater. However, other investigations observed no differences in HR between exercise modes (Bishop, Frazier, Smith, & Jacobs, 1989; Michaud, Rodriquez-Zayas, Andres, Flynn, & Lambert, 1995; DeMaere & Rudy, 1997). Factors such as familiarity with deep water (Frangolias & Rhodes, 1995), altered running technique (Wilber & Brennan, 1993), differences in water temperature (Brown et al., 1996), and exercise protocol (DeMaere & Rudy, 1997) have contributed to this discrepancy in the literature. DeMaere and Rudy (1997) tested the acute metabolic responses of cross-country runners who were currently incorporating deep water running into their training routines.
CONCLUSION
Our data indicate that pulmonary function at rest may be compromised in the water compared to that measured out of the water in male, but not in female, swimmers. The decrease in respiratory function may have a negative effect on the swimming performance. A possible alteration in pulmonary function during exercise in the water compared to that measured out of the water for either gender is yet to be elucidated. In any case, the optimal function of the respiratory system may be minimizing the potential negative effect of water submersion on swimming performance.
REFERENCES
Agostoni, E.G., Gurtner, G., Torri, G., & Rahn, H. (1966). Respiratory mechanics during submersion and negative-pressure breathing. Journal Applied Physiology, 21, 251-258.
Andrew, G.M., Beecklake, M.R., Guleria, J.S., & Bates, D.V. (1972). Heart and lung functions in swimmers and non athletes during growth. Journal Applied Physiology, 32, 245-251.
Bishop, P.A., Frazier, S., Smith, J., & Jacobs, D. (1989). Physiologic responses to treadmill and water running. Physiology Sports Medicine, 17, 87-94.
Blimkie, (1992). Resistance training during pre- and early puberty: Efficacy, trainability, mechanisms and persistence. Canadian Journal of Sports Sciences, 17, 264-279.
Borg, C.V. (1982). Psychophysical basis of perceived exertion. Medicine and Science in Sports and Exercise, 14, 377-387.
Boutellier, U., Buchel, R., Kundert, A., & Spengler, C. (1992). The respiratory system as an exercise limiting factor in normal trained subjects. European Journal Applied Physiology, 65, 347-353.
Brown, S.P., Chitwood, L.F., Beason, K.R., & McLemore, D.R. (1996). Perceptual responses to deep-water running and treadmill exercise. Motor Skills, 83, 155-162.
Bye, P.T.P., Farkas, G.A., & Roussos, C. (1983). Respiratory factors limiting exercise. Annual Review Physiology, 45, 439-451.
Christie, J.L., Sheldahl, L.M., Tristani, F.E., Wann, L.S., Sagar, K.B., Levandoski, S.G., Ptacin, M.J., Sobocinski, K.A., & Morris, R.D. (1990). Cardiovascular regulation during head-out water immersion exercise. Journal Applied Physiology, 69, 657-664.
Chu, K.S. & Rhodes, E.C. (2001). Physiological and cardiovascular changes associated with deep water running in the young-possible implications for the elderly. Sports Medicine, 31, 33-46.
DeMaere, J.M., & Rudy, B.C. (1997). Effects of deep-water and treadmill running on oxygen uptake and energy expenditure in seasonally trained cross-country runners. Journal Sports Medicine Physical Fitness, 37, 175-181.
Dempsey, J.A., Johnson, B.D., & Kurt, W. (1990). Adaptation and limitations in the pulmonary system during exercise. Chest, 97 (Suppl 3), S81-S87.
DiPrampero, P.E. (1986). The energy cost of human locomotion on land and in water. International Journal Sports Medicine, 7, 55-72.
Edlund LD, French RW, Herbst JJ, Ruttenburg HD, Ruhling RO, & Adams TD. (1986). Effects of a swimming program on children with cystic fibrosis. American Journal Disease Child, 140(1), 80-3.
Engstrom, I., Ericksson, B.O., Karlberg, P., Saltin, B., & Thoren, C. (1971). Preliminary report on the development of lung volumes in young girl swimmers. Acta Paediatric Scandinavica Supplement, 21, 73-76.
Frangolias, D.D., & Rhodes, E.C. (1995). Maximal and ventilator threshold responses to treadmill and water immersion running. Medicine Science Sports Exercise, 27, 1007-1013.
Harms, C.A., Wetter, T.J., St Croix, C.M., Pegelow, D.F., & Dempsey, J.A. (2000). Effects of respiratory muscle work on exercise performance. Journal Applied Physiology, 89, 131-138.
Keens, T.G., Krastins, J.R.B., & Wannamaker, E.M.. (1977). Ventilatory muscle endurance training in normal subjects and patients with cystic fibrosis. American Review Respiratory Diseases, 116, 853-860.
Lomax, & McConnell (2003). Inspiratory muscle fatigue in swimmers after a single 200 m swim. Journal Sports Sciences, 21, 659-664.
McArdle, W., Katch, F., & Katch, V. (2000). Essentials of Exercise Physiology. Anaerobic and Aerobic Training. Lippincott Williams & Wilkins. 2rd Edition, USA.
Michaud, T.J., Rodriquez-Zayas, J., Andres, F.F., Flynn, M.G., & Lambert, C. (1995). Comparative exercise responses of deep-water and treadmill running. J. Strength Conditioning Research, 9, 104-109.
O’Kroy, J., Loy, R., & Coast, R. (1992). Pulmonary function changes following exercise. Medicine and Science in Sports and Exercise, 24, 1359-1364.
Ritchie, S.E., & Hopkins, W.G. (1991). The intensity of exercise in deep-water running. International Journal Sports Medicine, 12, 27-29.
Sambanis M. (2006). Effects of detraining on pulmonary function and performance in young male swimmers. Minerva Pneumologica, 45, 121-128.
Vaccaro, P., & Clarke, D.H. (1978). “Cardiorespiratory alterations in 9 to 11 year old children following a season of competitive swimming. Medicine and Science in Sports, 10(3).
Volianitis, S., McConnell, A.K., Koutedakis, Y., McNaughton, L., Backx, K., & Jones, D.A. (2001). Inspiratory muscle training improves rowing performance. Medicine Science in Sports and Exercise, 33(5), 803-809.
Vrabas, I.S., Dodd, S.L., Powers, S.K., Hughes, M., Coombes, J., Demirel, H., & Reid, M.B. (1999). Endurance training reduces the rate of diaphragmatic fatigue in vitro. Medicine Science in Sports and Exercise, 31(11), 1605-1612.
Wenger, H.A., & Bell (1986). The interaction of intensity, frequency, and duration of exercise training in altering cardiorespiratory fitness. Sports Medicine, 3, 346-356.
Wilber, R.L., & Brennan, D.K. (1993). Physiological responses to deep-water running in athletes. Sports Medicine, 16, 374-380.
Zinmam, R., & Gaultier, C. (1986). Maximal static pressures and lung volumes in young female swimmers. Respiratory Physiology, 64, 229-239.